Cervical Fusion Cage System Interbody Spine Implant
The Cervical Fusion Cage System Interbody Spine Implant is inserted anteriorly into the cervical spine after discectomy. The device is designed to maintain intervertebral height, preserve the space between vertebral bodies, hold bone graft material, and support the restoration of cervical lordosis.
The implant consists of biocompatible materials that allow stable placement between the cervical vertebrae. It may be combined with standard anterior cervical fixation instruments when required. Its structural design helps support load-sharing and provides the stability needed for the bone fusion process.
Anatomically Contoured Design: Less damage to the endplate as a result of the implantation.
Large Central Graft Window: Facilitates the use of autograft or allograft bone.
Multiple Height and Lordosis Angle Options: Assists the restoration process of cervical lordosis.
Blunted Leading End Design: Reduces the pulling of soft tissue during insertion.
Anti-Migration Surface Structure: Enhances the very first mechanical stability.
Optional use of radiographic markers or metal coating: Makes post-operative imaging more convenient.
The Cervical Fusion Cage System Interbody Spine Implant is placed anteriorly during cervical fusion surgery to provide structural support between vertebral bodies. Typical indications include:
Degenerative cervical disc disease
Reconstruction after discectomy for herniated disc
Loss of intervertebral height associated with cervical spondylosis
Foraminal narrowing resulting from disc space collapse
Cases requiring interbody support during anterior cervical fusion
It can be used together with standard anterior cervical surgical instrument sets to support the overall procedure.
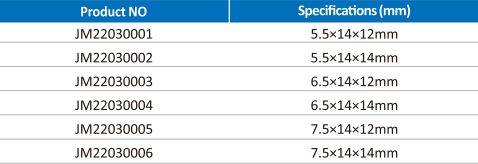
Specification
| Brand | JinMing Medical |
|---|---|
| Material | PEEK |
| Size Options | Multiple footprints, heights, and lordotic angles |
| Graft Chamber | Large central cavity for graft packing |
| Design Features | Anti-migration structure; clear radiographic markers |
| Certificate | CE, ISO 13485 |
| OEM / ODM | Available |
Why Choose JinMing Medical's Cervical Fusion Cage System Interbody Spine Implant?
Global Trust: Medical professionals in more than 60 countries rely on a partner they can trust.
Expertise & Precision: A knowledge of over 20 years in the field of orthopedic mplements and instruments is combined with the utilization of 5-axis CNC and ZEISS CMM for ±0.01 mm accuracy.
Quality Without Question: Backed by CE, ISO 13485, and 50+ international certifications—so you never have to doubt.
A Legacy of Safety: Every product is 100% inspected, supporting a flawless zero-medical-incident record for complete peace of mind.
Customization is Our Expertise: We've brought over 2,000 unique SKUs to life through full OEM/ODM and co-development partnerships.



Certifications & Quality Assurance
Internationally Certified: All products meet CE and ISO13485 international quality standards.
100% Quality Inspected: Every product undergoes full inspection to guarantee its safety and performance.
Complete Customization: We provide OEM/ODM services for screw angles, plate sizes, and labels tailored to your specific needs.
