Interbody Fusion Cage Titanium Mesh Spine Implant
The Interbody Fusion Cage Titanium Mesh Spine Implant is used in spinal fusion procedures. It helps maintain vertebral height and provides a chamber for bone graft material, supporting the biological environment needed for fusion.
The open mesh architecture allows bone graft material to interdigitate through the titanium mesh and fill the defect while allowing load-sharing contact with surrounding bone after reconstruction.
This device is commonly used for anterior column reconstruction in spinal fusion procedures where structural support and graft accommodation are required.
High-Quality Titanium Mesh
Provides a rigid structure capable of sharing load in vertebral reconstruction.
Large Central Bone Graft Area
Allows packing of autograft or allograft bone.
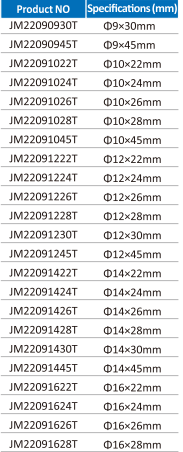
Multiple Heights and Diameters
Accommodates vertebral defects of varying sizes.
Textured Outer Surface
Designed to enhance initial mechanical stability after implantation.
Radiopaque Markers
Assist with intraoperative and postoperative imaging.
Adjustable Tube-Style Mesh
Allows trimming or length adjustment according to clinical requirements.
Corrosion-Resistant Titanium Alloy
Suitable for long-term spinal implantation.
These titanium mesh interbody devices are commonly used for:
Anterior column reconstruction after vertebrectomy
Interbody fusion requiring structural support
Stabilization as part of reconstruction for traumatic vertebral defects (when used with appropriate anterior fixation)
Restoration of vertebral height loss due to degeneration
Reconstruction of vertebral defects associated with tumors
They may be used in combination with standard spinal fusion instrument sets during surgical procedures.
Specification
| Brand | JinMing Medical |
|---|---|
| Material | Titanium Alloy |
| Structure | Cylindrical or anatomical titanium mesh with open lattice design |
| Size Options | Multiple heights and diameters; length can be trimmed as required |
| Graft Chamber | Large central cavity for effective bone graft packing |
| Design Features | Radiopaque markers, trimmable mesh, stable load-sharing design |
| Certificate | CE, ISO 13485 |
| OEM / ODM | Available |

JinMing Medical's Interbody Fusion Cage Titanium Mesh Spine Implant Advantages
60+ Countries Served: A truly global partner in orthopedic innovation.
500,000+ Products Delivered: A legacy of scale and reliability.
Zero Quality Incident Lawsuits: The ultimate testament to our quality and safety.
2,000+ Custom SKUs Developed: Proven expertise in OEM/ODM and co-development.
5,000 Units Daily Peak Capacity: The capability to meet your demand, reliably
Customization is Our Expertise: We've brought over 2,000 unique SKUs to life through full OEM/ODM and co-development partnerships.



Our Certifications & Quality Assurance

Let's Shape the Future of Orthopedic Medicine Together.